
The Autologous Fat, Breast Augmentation Effectiveness Trial is a prospective study comparing routine fat grafting versus Stromal Vascular Fraction enriched fat grafting
Protocol identifying number: 0001
Principal investigator:
James P. Watson, MD.
Associate Clinical Professor, UCLA
1301 20th Street, Suite 470
Santa Monica, CA 90404, USA
Phone: 310–829–6876
Fax: 310–362–8449
Co-investigators:
Associate Clinical Professor, USC
1301 20th Street, Suite 470
Santa Monica, CA 90404
Gerald M Pohost, MD
Professor, Keck School of Medicine
Professor, Viterbi School of Engineering
University of Southern California
Director of Research, Hollywood Presbyterian Medical Center
1300 N. Vermont Avenue, Suite 610
Los Angeles, CA 90027
Sponsor information:
CHA Hollywood Medical Center, L.P.
1300 North Vermont Avenue
Los Angeles, CA 90027
Phone: 310–413–3000
Fax: 00000000
Address of research site
Hollywood Presbyterian Medical Center
1300 North Vermont Avenue
Los Angeles CA 90027
Tel: (213) 413–3000
Abbreviations
ADSC, Adipose Derived Stem Cell MRI, Magnetic Resonance Imaging
AFG, Autologous Fat Grafting OR, operating room
ASPS, American Society of Plastic Surgeons PBS, phosphate buffered saline
ECG, Electrocardiography PPE, physician physical exam
HBSS, Hanks Balanced Salt Solution SP, standard photography
HBV, Hepatitis B Virus SVF, Stromal Vascular Fraction
HIV, Human Immunodeficiency Virus VM, volumetric measurements
PURPOSE OF THE STUDY
Autologous fat grafting (AFG) for cosmetic breast augmentation is now an acceptable alternative to saline or silicone gel implants in the US. The American Society of Plastic Surgeon’s (ASPS) Task Force on Fat Grafting has issued a consensus statement in support of the safety and efficacy of AFG for breast augmentation. (See Appendix A) Outside the US, AFG for breast augmentation is also an acceptable alternative to breast implants but in these countries there is mounting evidence that enriching AFG with adipose derived stromal vascular fraction (SVF) increases the efficacy of AFG breast augmentation. Despite widespread use of AFG alone and SVF-enriched AFG, no clinical trial has compared these two methods. For this reason, both current and past presidents of the American Society of Plastic Surgeons (ASPS) have called for a prospective, randomized study to be done to produce “Level I” or “Level II” evidence that SVF-enriched AFG is superior to AFG alone. The American Society of Aesthetic Plastic Surgeons (ASAPS), the ASPS Task Force on Fat Grafting, and the International Federation of Adipose Therapeutics and Science (IFATS) have all indicated for such a study to be done as well, yet such a study has not yet been performed. For this reason, the purpose of this study is to objectively compare outcomes between AFG breast augmentation vs. SVF-enriched AFG for cosmetic breast augmentation in a carefully designed study that will provide both Level I and Level II evidence for the superiority of SVF-enriched AFG over AFG alone, or prove that there is no difference between the two methods. This study is carefully designed to give the patient the option of enrolling in either the Level I or Level II study. Therefore this proposal is a two-armed study with a contra-lateral control, level I study in one arm, and a cohort control, level II study in the other arm.
SPECIFIC AIMS:
1. To compare outcomes between autologous fat grafting(AFG) versus autologous fat grafting enriched with adipose derived stromal vascular fraction (SVF) for breast augmentation.
2. To evaluate the MRI appearance of fat grafting with SVF versus fat grafting without SVF.
3. To evaluate the technique of proton MR Spectroscopy and 3D IDEAL fat-water MRI in comparing lipid content and water-fat fractions of AFG with and without SVF.
4. To define differences in indications, candidates, and populations that would benefit from fat grafting for breast enhancement versus traditional prosthetic/traditional augmentation.
5. To evaluate short term and long term survival of fat grafting versus SVF enriched fat grafting at one month, 6 months, and one year.
6. To evaluate patient satisfaction with the outcome of these procedures.
RESULTS/REFERENCES OF PREVIOUS RELATED RESEARCH
Autologous Fat Grafting (AFG) to the Breast (without SVF) The first 127 references in the bibliography of this proposal relate to the subject of autologous fat grafting. The following paragraphs summarize those references.
Autologous fat grafting (AFG) for breast augmentation has been around for over 110 years but was not accepted or endorsed by organized plastic surgery until the last decade (beginning in 2000). The primary two reasons for this long delay are 1) unpredictable outcomes and 2) artifacts present on mammography. The history of AFG goes back to 1895, when Dr. Vincenz Czerny used a lipoma from an opera singer’s lumbar area to fill a breast defect from a tumor removal. AFG to the breast did not exist, however, until the advent of suction assisted lipectomy (liposuction) developed by Gerald Illouz and others in the early 1980s. Although liposuction was initially developed for fat removal, it soon became evident that this was a simple, efficient way to harvest fat for grafting purposes. Within a few years, fat harvesting via liposuction became popular for contour deformity repairs, facial aging, and breast deformities. Although fat grafting quickly became acceptable for contour deformity correction and facial fat grafting, there were many who voiced concerns about injecting the breast with fat. It was recognized that transplanted fat would die, leaving behind scarring and microcalcification. Many were concerned that this would be detrimental to mammographic screening for breast cancer. By the mid 1980s, the American Society of Plastic Surgeons became concerned enough with the safety of breast AFG that they formed a task force to study this issue. This ASPS task force speculated that fat grafting caused calcifications of the breast, and contemporary studies clearly proved them correct. As a result, the task force formulated a position paper, predicting that fat grafting would compromise breast cancer detection; although no research evidence other than level IV evidence was available to support these predictions. Their consensus was that AFG to the breast should be contraindicated. This position paper was published in 1987. As a result, the plastic surgery community largely avoided AFG to the breast during the 1990s and early 2000s.
A few US surgeons did not agree with the 1987 ASPS task force position paper. Their basis for this was that studies of breast reduction patients showed similar mammographic abnormalities, yet breast reduction procedures were not prohibited due to this effect. Brown et al presented a retrospective study showing that calcifications were present in 50% of mammograms performed 2 years post breast-reduction surgery (Brown FE et al., 1987). Despite this evidence, the ASPS task force did not suggest that breast reduction surgery be contraindicated or eliminate the voluntary ban on autologous fat grafting to the breast. These surgeons clearly pointed out the double standard that had been adopted by ASPS in which fat grafting to the breast was judged by a completely different standard than breast reduction. In addition, radiologists started re-examining microcalcifications due to previous breast reduction and better standards were developed for grading mammographic abnormalities by the American College of Radiology (the BIRADS system). As a consequence, radiologists became more adept at distinguishing the differences between post surgical microcalcifications and de novo calcifications caused by cancer. This new mammographic standard largely eliminated the last pillar of evidence supporting the contraindication of AFG to the breast that was made by the ASPS task force in 1987. These surgeons, including Sidney Coleman, continued to develop more reliable techniques for AFG. In other countries no attempt (by plastic surgery societies) was made to prohibit AFG to the breast. As a consequence, this was noncontroversial and became popular in Europe and Asia. During this time, over 110 articles on fat grafting have been published in the scientific literature. Numerous other studies support the idea that radiologists have a high level of confidence in differentiating between fat necrosis calcifications after breast surgery and those related to breast cancers.( Kneeshaw PJ et al., 2006; Ynud M et al., 2004).
A new ASPS Task Force on fat grafting was convened in 2006 to re-evaluate the safety of AFG to the breast. All of the scientific articles were exhaustively reviewed and categorized by their level of scientific significance by a new ASPS Task Force on Fat Grafting that was convened in 2006 (See Appendix A). A new consensus paper was written by this ASPS Task Force in February, 2009. This consensus paper was based on sound scientific evidence that had emerged from the 110 publications on AFG. Their consensus was that AFG to the breast was safe, but that more research was needed with a prospective Level I or Level II protocol. For this reason the design of is proposed as follows:
1. A small, Level I study comparing AFG to SVF-enriched AFG in the same patient. One side will be treated with AFG and the other side with SVF-enriched AFG. This study will be double-blinded, randomized by breast, and prospective. The AFG breast will be the control and the SVF-enriched AFG breast will be the experimental side (although this technique no longer considered experimental in all countries except the US).
2. A larger, Level II study comparing AFG to SVG-enriched AFG in different patients.
In this study, the patient can choose to have AFG in both breasts or SVF-enriched AFG in both breasts. The two patient groups will be compared over the same time period as in #1. Here the group with AFG in both breasts will be the control and SVF-enriched AFG in both breasts will be the experimental group.
Improving Autologous Fat Graft Survival
While the ASPS task force voluntarily contraindicated AFG breast augmentation was in effect in the US, AFG became a very popular procedure in other countries. The results of these procedures showed that the most common problem with AFG to the breast was fat graft resorption and loss of volume, not interference with mammography, as predicted by the ASPS task force. Research was performed to try to improve fat graft survival:
1. Less traumatic fat harvest — Many studies suggested that syringe based liposuction was less traumatic than mechanical liposuction. As a result, the harvest of fat for AFG
largely became a manual, syringe-based procedure internationally, and was also adopted by US surgeons such as Sidney Coleman who continued AFG procedures in the US during the 1990s.
Machine Aspiration

Syringe Aspiration

2. Density gradient separation of fat from blood and oil — With all forms of liposuction, a mixture of blood, fat, and disrupted fat (that looks like oil) occurs. Many researchers showed that graft survival and volume maintenance was not improved with the grafting of all of these components. As a consequence, several methods for separating the fat from the blood and oil were developed including Telfa-rolling, layering of the blood/fat/oil with gravity and then removing the blood and oil layers, as well as centrifugation. Centrifugation has emerged as the most popular method of separating the fat from the blood and oil. This method is now referred to as “density gradient” separation (FDA terminology), and is depicted below.

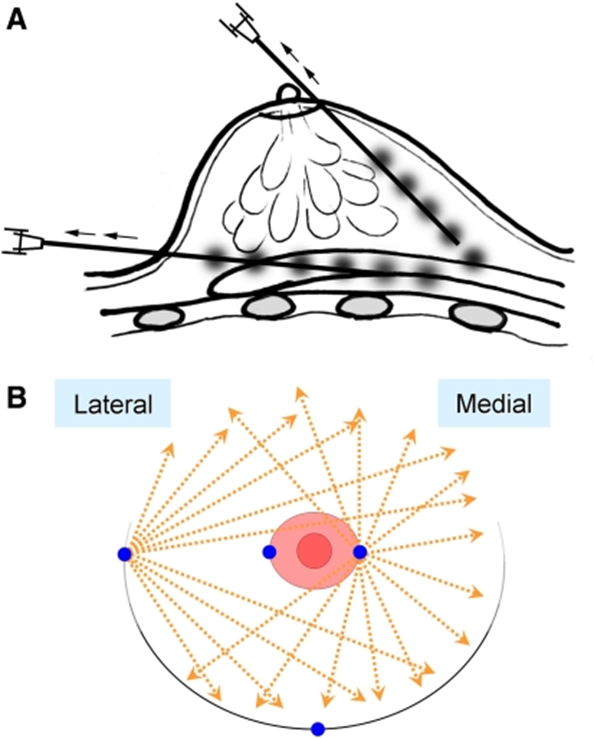
3. Injection of fat with small, 1mm tunnels in crisscrossing pattern, avoiding large “lumps” of injected fat. These small, 1mm diameter blunt tipped injection cannulae are usually called “Coleman canulas” and this technique referred to as the Coleman technique. The figures below show this injection technique for the breast.
Figure A — This diagram shows how the fat is injected above and below the actual breast parenchyma. The fat grafts are actually NOT injected directly into the breast tissue, since the breast tissue is very dense.
Figure B — This diagram shows how multiple tunnels are created from the edge of the breast and from the edge of the areola. These tunnels are all made with small, blunt-tipped 1mm diam. cannulae to avoid any bleeding/trauma to the fat compartment. One millimeter “beads” of fat are in-jected into each tunnel.
4. Washing the aspirated fat — Other researchers believed that washing the syringe aspirated fat would further improve survival. Despite many studies on this technique, no emerging evidence exists to show increase in fat survival with fat washing.
5. Stromal vascular fraction enrichment — When adipose-derived stem cells were discovered in 2000 by Zuk, et al.,this news instantly became of interest to clinicians doing AFG. This led to the development of SVF enriched fat grafting in many countries, but especially in Japan. This is discussed in detail below.

Stromal Vascular Fraction Enrichment of Fat Grafting
The discovery of mesenchymal stem cells in adult fat by Zuk, et. al. in 2000 led to an explosion in research over the past 10 years. These adipose-derived stem cells have been shown to be pleuripotent and have been differentiated into multiple cell lines, including bone, muscle, nerve, and hepatic cells. The discovery of these cells was made by using density gradient separation of fat from the blood and oil (as described in the previous section), enzymatically breaking down fat globules into individual cells, and then washing (neutralizing) the enzymes out of the cell suspensions. These steps provided the researcher with a heterogeneous mixture of cells from the fat stroma, rich in stem cells and vascular cells from the fat. As a result, this heterogeneous mixture became known as “stromal vascular fraction” or SVF. In the research laboratory, the SVG would be cultured and further cell separation would occur due to the adherence of ADSCs on the surface of the plastic flasks. However, cell culturing had several logistical and undesirable features for clinical use of the SVF.
1. Same day SVF use with AFG — Culturing did not allow the SVF to be used on the day of liposuction, eliminating the possibility of same-day, point-of-service procedures using AFG.
2. Keeping the “vascular” fraction — Culturing also eliminated the “vascular” from the “stromal vascular fraction.” Histologic studies showed that many of the cells in the SVF were vascular progenitor cells (pericytes, endothelial cells), which would be beneficial for revascularizing fat grafts.
3. Avoiding cell contamination/infection- Culturing also introduces the issue of bacterial or viral contamination when cells are grown over a period of weeks. By eliminating cell culture and using the SVF within 2–3 hours of liposuction, the risk of bacterial or viral infection during cell culture is also eliminated, making immediate SVF use a much lower risk.
Because of these advantages, many researchers started using SVF to enrich fat grafts for clinical use in facial fat grafting, breast fat grafting for breast reconstruction and cosmetic breast augmentation. As of today, over 100 manuscripts have been written and published in peer reviewed journals covering this method of SVF use.
Studies of SVF-enriched AFG for Breast Augmentation
Fat grafting to the breast for cosmetic breast augmentation is a promising treatment that has been tried many times by plastic surgeons, but has always suffered from unpredictable results, primarily due to a low rate of graft survival. Although the efforts described above (traumatic fat harvest, density gradient separation of blood and oil from the fat, the use of Coleman cannulae, and the Coleman injection technique) work well for the face, where only 10–30cc of fat is usually injected, with the breast 200–500cc of fat is required to make a difference in volume. To increase fat survival with these large volumes of fat grafting, a Japanese researcher has developed a novel strategy known as cell-assisted lipotransfer (CAL). In CAL, autologous adipose-derived stem (stromal) cells (ASCs) are used in combination with lipoinjection. A stromal vascular fraction (SVF) containing ASCs is freshly isolated from half of the aspirated fat and recombined with the other half. This process adds adipose derived stem cells and stromal vascular cells to the fat grafts, enriching the grafts with cells that can revascularize the fat. In a study of 40 patients who underwent CAL for cosmetic breast augmentation, Yoshimura was able to show consistently reliable breast augmentation by 100 to 200 ml volume after a mean volume of fat of 270 ml was injected. There was very little long term loss in volume and complications i.e., cyst formation and microcalcificationsonly occurred in 4 patients. Almost all the patients were satisfied with the soft and natural-appearing augmentation. This small study suggests that SVF enrichment of fat grafts increased survival of the fat dramatically compared to non-enriched fat grafting to the breast.
Non-enriched AFG to Breast

SVF-enriched AFG to Breast

These two diagrams illustrated the differences between autologous fat grafting (alone) on the left vs stromal vascular fraction (SVF) enriched fat grafting on the right. Here in “step 3”, half of the fat is used for SVF preparation and the other half of the fat is kept for grafting. The SVF and fat are mixed in “step 4” and then injected into the breast. This effectively doubles the concentration of stromal vascular fraction cells, which includes adipose derived stem cells.
I. CRITERIA FOR SUBJECT SELECTION
1. Number of research participants.
10 participants will be enrolled in the randomized, blinded, contra-lateral study where one breast will be treated with fat grafting, via the Coleman technique and the other breast will be treated with SVF enriched fat grafting. For patients who do not want to be randomized, we will enroll a total of 100 in the non-randomized, prospective study where the patient can choose to have either fat grafting alone (to both breasts) or SVF-enriched fat grafting (to both breasts).
2. Gender and age of research participants
Females that are ages 18–35, with no prior history of breast surgery or breast cancer or augmentation of breast volume, biopsy or cyst. The reason for this decision is due to the low risk of breast cancer in this age group, thereby avoiding any issue of breast cancer occurrence being causally related to SVF-enriched AFG.
3. Racial and Ethnic Origin.
There is no restriction of racial and ethnic origin for this research.
4. Inclusion Criteria.
In this study, all patients will be included provided they meet the following inclusion criteria.
4.1
Patients must consent in writing to participate in the study by signing and dating an informed consent document indicating that the patient has been informed of all pertinent aspects of the study prior to completing any of the screening procedures.
4.2
Female of any race, age 18–35.
4.3
Patients must desire having a small breast augmentation with a ½–1 cup size increase in their breast volume, not a 1–2 cup size increase in breast size. (If they wish to have a larger breast size increase, then they must understand that a second procedure may be required to achieve that size increase or that they would be better served with a traditional saline or silicone gel breast augmentation).
4.4
Patients must have a normal physical exam with no breast masses, no nipple discharge, no fibrocystic disease, no axillary adenopathy and/or history of abnormal bleeding.
4.5
Patients must not be pregnant or lactating when enrolled in the study and must agree to have a pregnancy test (urine or blood) prior to the surgical procedure. The patient should also not be trying to get pregnant during the course of the study.
4.6
In the absence of contraindication patients enrolled in the level I (randomized study) must also agree to have a pre-operative and post op MRI after the procedure is completed.
4.7
Patients must also consent to be photographed with conventional photography as well as 3D imaging before and after the procedure and at the end of the study.
4.8
All patients in the level I (randomized) and level I (nonrandomized) part of the study must also consent to undergo ultrasound imaging of the breast prior to the procedure and after the procedure to evaluate and treat any oil-filled cyts that may arise due to the procedure.
4.9
All patients in the level I (randomized) and level II (nonrandomized) part of the study must also consent to allow their results to be included in any scientific research, scientific publication, or presentations at scientific meetings.
4.10
All patients in the level I and level II part of the study must also consent to allow their photos to be used for publication in scientific journals, internet website, and patient educational material such as brochures, before and after photos, and patient education videos.
4.11
All patients in the study must also agree to have mammography done when indicated at any time after the procedure.
4.12
All patients in the study must agree to have a biopsy of any suspicious abnormality seen on mammography, ultrasound, or MRI during the study.
4.13
Patients must not be anemic at the time of the procedure (Hg < 10). If they are anemic at the time of enrollment, they can still be included in the study if the anemia is corrected pre-operatively with iron supplementation. This must be continued during the study to avoid any adverse reporting that may not be related to the procedure (i.e. anemia not caused by SVF-enriched fat grafting)
4.14
Patients must have no history of abnormal bleeding during surgery, bruising and no personal/family history of coagulopathy.
Patients must be off aspirin and other NSAIDs for two weeks prior to the procedure and have a normal blood coagulation panel within two weeks of surgery: Pts > 150K, PT(INR) < 1.3, APTT <1.3×control.
4.15
Patients must have normal renal function (demonstrated by a Creatinine ≤ 1.5 mg/dl,) and no urinary tract infection (less than 5 WBCs on UA and leukocyte esterase negative on U/A Dipstick). If a UTI is present, the patient may still enroll if this is treated pre-operatively
4.16
Patient must have no personal history of breast cancer. If a multigenerational family history of breast cancer or ovarian cancer is present, the patient must be first evaluated by a surgical oncologist or medical oncologist to obtain pre-operative clearance and reassurance that they do not need BRCA testing.
4.17
Patients with prior radiation to the chest wall (for Hodgkins disease) may not be included in the study.
4.18
Patients must have a stable weight and not be fluctuating in their weight (otherwise this will distort volumetric measurements of breast size post operatively, making it impossible to determine true augmentation volume from SVF-enriched AFG.
5. Exclusion Criteria.
Patients presenting any of the following will be excluded from the study:
5.1
Patients with a positive pregnancy test
5.2
Patients with an abnormal breast exam
5.3
Patients with a bleeding disorder who are on anticoagulants
5.4
Patients with existing breast implants, saline or silicone gel
5.5
Patients unwilling to consent to the procedure, photography, MRI, ultrasound, or unwilling to come back for the required follow-up testing and evaluation
5.6
Patients with a prior history of breast cancer
5.7
Patients with a contraindication for MRI (e.g. pacemaker, ICD, etc)
5.8
Patients with a positive BRACA1 or BRCA2 gene mutation
5.9
Patients who are anemic despite iron supplementation and treatment of the underlying cause of the anemia.
5.10
Patients unwilling to have mammographic testing done before and after the procedure
5.11
Patients with Fibromyalgia, regional pain syndrome, or chronic fatigue.
5.12
Patients with positive human immunodeficiency (HIV), hepatitis B (HBV) or hepatitis C (HCV) at screening indicative of current of pass infection.
5.13
Patients with a bleeding diathesis
5.14
Patients with a positive urinalysis for pregnancy or UTI.
5.15
Patients whose diabetes is not adequately controlled (HgA1c > 7).
5.16
Patients with a history of local anesthetic allergy.
5.17
History, diagnosis, or signs and symptoms of clinically significant psychiatric disorder, including but not limited to
● Severe psychological disease (e.g. schizophrenia, manic-depressive disorder, severe depression, etc).
● Body dysmorphia syndromes, i.e., anorexia, bulimia, etc.
● Somatoform disorders
.
5.18
Potential research participants with a substance abuse (e.g. alcoholism, medical narcotics (morphine, Vicodin, codeine, Percodan, etc.) illicit drug, prescription medications without a valid prescription, etc.) within 2 years of screening, and tobacco habit.
5.19
Alanine aminotransferase (ALT) or aspartate aminotransferase (AST) ≥ 3.0 times the upper limit of normal, or creatinine exceeding 1.7 mg/dL (150 mol/L) in men or 1.5 mg/dL (133 mol/L) in women, hemoglobin ALC ≥ 10% at screening.
5.20
Research participants who are or have been receiving immunosuppressants such as Cyclosporin A or azathioprine within the past six weeks
5.21
Research participants on anticoagulants which cannot be stopped or corrected.
5.22
Use of biologics, e.g. TNF-inhibitors, such as adalimumab, etanercept, infliximab, including any live vaccines within 3 months of the initial pain assessment period.
5.23
Research participants who are taking anticoagulants such as coumadin, fixed dose, non fractionated heparin or low molecular weight heparin (Lovenox).
5.24
Oral or parenterally administered systemic corticosteroids within 30 days prior to the initial pain assessment period.
5.25
Individuals largely or wholly incapacitated, e.g. bedridden or confined to a wheelchair, permitting little or no self-care.
5.26
Pregnant women, lactating mothers, women suspected of being pregnant, women who wish to be pregnant during the course of the clinical study.
5.27
Research participants who are presently or have been enrolled in other clinical trials within the past four weeks.
5.28
Research participants who the principal investigator considers inappropriate for the clinical trial due to any other reasons than those listed above.
Vulnerable Subjects
No vulnerable subjects (e.g., those with limited autonomy or those in subordinate hierarchical positions) will be included. Children, pregnant women, nursing home residents or other institutionalized persons, students, employees, fetuses, prisoners, and persons with decisional incapacity will not be included in this study.
Recruitment, enrollment, and renumeration
Recruitment of volunteers into the study will be done via referral from clinics, referral from physicians caring for patients with osteoarthritis, as well as direct public service announcements through print media, direct mail, and e-mail announcements. No compensation will be given to referring physicians, to patient volunteers, or to any referral service.
Marketing
Marketing of the breast augmentation program will be done through Hollywood Presbyterian Medical Center’s marketing department with posters and public service announcements through print media, direct mail, and e-mail announcements, TV, and the internet.
Volunteer compensation
No compensation will be given to patient volunteers who are entered into the experimental group or the control group of patients.
II. METHODS AND PROCEDURES
The proposal will be a prospective, two armed study. Patients will be given the option (freedom of choice) to enroll in a Level I study where they will serve as their own “contra-lateral control”. Here one breast will be treated with fat grafting only and the other breast will be treated with SVF-enriched fat grafting of an identical volume. If the patients enroll in this portion of the study, their SVF harvest, processing, and fat enrichment at no charge (i.e. this portion of the cost of the procedure will be free).
If they are unwilling to enroll in the Level I study, they will be given the option of enrolling in the Level II study where both breasts will be treated similarly with SVF-enriched fat or alternatively both breasts will be treated identically with non-enriched fat grafting. Either way, the patient will have to pay for the full cost of the procedure for this non-randomized portion of the study. Essentially, this will be an open-label study. Only those examining the patients post op and those viewing the photographs and 3D imaging studies will be blinded. The goal will be to enroll half of the patients in the SVF enriched AFG limb of the Level II study and half of the patients in the non-SVF enriched AFG limb of the Level II study.
No drug, biologic or dietary supplement or medical device will be involved in this study. Nor is there any form of gene transfer involved in this study. Furthermore, there is no known carcinogen or mutagen of any kind involved in this study.
- Patient evaluation. All patients will be evaluated with photographs, 3D imaging, mammography, ultrasound, MRI, history, and physical examination. Blood work including hemoglobin, hematocrit, WBC, pre-albumin, HgA1c, BUN, Creatinine, HIV, HBV, HCV, prothrombin time, partial thromboplastin time, liver function tests and comprehensive metabolic panel as well as pregnancy test. Additional work-up, prior to treatment, will be done for any abnormality found on breast examination, mammography, ultrasound, or MRI.
- Eligibility screening and Informed consent. Patients will then be fully informed of research protocol and a determination will be made if they fit within the inclusion criteria and do not fit within the exclusion criteria. The health care professional explaining the study and consent form to the patient, and if the patient is willing, the informed consent forms will be signed by the patient.
- Harvest of adipose tissue — Adipose tissue harvest will be done under general anesthesia in the operation room (OR) by a Board certified or Board eligible plastic surgeon with hospital privileges to do liposuction at Hollywood Presbyterian Medical Center. The surgeon will have to be proctored by either Dr. James Watson or Dr. Joel Aronowitz for their first 5 cases of autologous breast augmentation with SVF-enriched fat or the control procedure, which will consist of autologous fat grafting without SVF-enrichment. All of the anesthesia will be administered by a board certified or board eligible anesthesiologist. All patients will then be taken to the (OR) for the procedure of liposuction. This will be done under sterile conditions. Lipoaspirate will be harvested using syringe liposuction technique with Tumescent local anesthesia (see Appendix B for technique details and physician training requirements). These tissues will then be processed as described below under section 4. Briefly, local anesthetic (lidocaine 1% with 1/100,000 epinephrine and Marcaine 0.5% with 1/200,000 epinephrine) solution will be injected into the adipose tissues. A 3mm blunt cannula attached to a syringe will be used to extract fat tissue. Minimal negative pressure will be applied by the piston action of the plunger. A minimal volume of 300cc of lipoaspirate will be harvested. Syringe liposuction harvest will be stopped when the lipoaspirate coming out of the syringe starts to tur bloody, indicating that bleeding is occurring. This “end point” will determine the volume of fat that can be safely and successfully harvested (not an arbitrary number).
- Processing of fat and extraction of SVF. The extracted fat will be processed via the technique developed by Yoshimura, at the University of Tokyo, Japan, and modified for reduced time of processing (with the exception that the cells in the infranatant solution will not be processed). Are we to include Reference 130,136????
Technique Summary:
A. Centrifuge the fat harvested from syringe liposuction — Take all of the fat harvested from the syringe liposuction procedure and put it in 50cc sterile plastic centrifuge tubes. Divide the fat into an even number of centrifuge tubes to balance the centrifuge rotor. Centrifuge the fat at 700g for 5 minutes, then remove the infranatant blood/tumescent fluid and the supranatant oil/disrupted fat. Set half of the remaining fat aside for re-injection and the process the other half of the fat for SVF use.
B. Prepare collagenase and collagenase neutralizing agent — Fresh collagenase will be prepared or frozen collagenase will be thawed out from the freezer where it is stored at — 4 C. Collagenase used will be GMP grade, non-animal source collagenase from VitaCyte, Indiana, USA. The product is a Blend 1 formulation, Cat. №005–1010. This is a mixture of collagenase I and collagenase II, derived from bacterial production (Clostridium histolyticum) and a neutral protease (derived from B. polymyxa). One vial of Blend 1 formulation will be used per 100gm of centrifuged fat processed. The enzyme activity of each vial is 25 Wunsch units for the collagenases and 200,000 neutral protease units for the B. polymyxa neutral protease.
The following is the protocol for collagenase preparation:
1. Pre-heat Hanks balanced salt solution (HBSS) in incubator at 37°C
2. Add 5ccs of pre-heated HBSS to 1 vial of collagenase (100 mg of enzyme) and
let this sit in incubator at 37°C.
3. Add the dissolved collagenase to 95cc of HBSS, giving you a total volume of
100cc of collagenase solution ready for mixture with fat (1:1 mixing ratio)
keeping this collagenase solution in incubator at 37°C.
4. Mix more vials of collagenase as described above, based on the volume of
lipoaspirate available for stem cell aspiration isolation. 100cc of collagenase
solution should be used for every 100cc of lipoaspirate set aside for SVF
preparation.
C. Washing adipose tissue for SVF preparation. Take half of the centrifuged fat from
step A and add an equal amount of saline. Centrifuge this solution for 700G for 5 minutes. Pipette out the infranatant solution and discard it. Pre-heat washed fat in incubator, allowing the fat to reach 37°C prior to step D.
D. Collagenase digestion. Mix equal volumes of the washed fat and collagenase prepared in Steps B and C. Incubate this solution in a shaker set at 200rpm for 30minutes at 37oC. The volume in each test tube should be 50 cc and the test tubes should be placed in the centrifuge in opposite bays to balance the centrifuge (i.e. 100cc, 200cc, 300cc, or 400cc total).
E. Post collagenase digestion centrifugation. Remove the collagenase/fat tubes from
the Shaker and centrifuge at 800G for 10 minutes at room temperature.
F. Collagenase neutralization — Remove fat from the supranatant layer of each
centrifuge tube and discard. Resuspend the lower layer by removing this with a pipette (25cc per new tube) and place this in new 50cc centrifuge tubes. Add 25cc of the patient’s own plasma so that the total volume per tube is 50cc. Then go through the following 3 cycles of centrifugation:
1. Centrifuge at 800G for 5 minutes at room temperature, and then remove supranatant (fat), and resuspend with neutral agent for final volume of 50cc per tube.
2. Centrifuge at 800G for 3 minutes at room temperature, and then remove
Supranatant (fat) and resuspend with neutral agent for a final volume of 50cc per tube.
3. Centrifuge at 800G for 3 minutes at room temperature, but do not discard
supranatant fat (see next step).
5. Quality Control measures — The following tests will be done to ensure the safety of the procedure by verifying cell number, bacterial contamination, cell viability, and % of cells that are actually stem cells (CD 34+ staining).
A. Cell count — We will count the cells using a hemacytometer and the Invitrogen
Countes automated cell counter. Record the total cell number and the number of cells between 9 μm and 15 μm (this is the size range of the stem cells. RBCs are smaller, WBCs are larger)
B. Cell culture — An aerobic culture will be taken of the cells and sent to the lab to
document that no contamination occurred during the SVF isolation procedure.
C. Cell Viability testing — Cell viability will be done with tryphan blue staining and Examination under a microscope (manual viability testing) and with the
D. Stem cell counts — A 100 μl sample of the SVF will be saved for future stem cell identification (for fluorescent antibody labeling for CD34+ markers). The 100 μm sample will be put in a 2.0 cc tube and 1.5 ml of cell banker (cell cryopreservation medium) will be added. This will be preserved at — 80 C and then batch tested with a fluorescent microscope and flow cytometry at a later date.
6. Prepare the adipostromal cells for injection — Take the leftover cell mixture add neutral agent to create a total volume of 50cc. Centrifuge this at 800G for 5 minutes. Remove the supernatant layer and now you have the SVF, ready for mixing with the preserved fat.
7. SVF and AFG mixing — Take SVF and the fat that was set aside into the OR. The surgeon will then mix the SVF with the fat and transfer this mixture into 10cc syringes using a Coleman, female-to-female transfer hub.
8. SVF-enriched fat grafting. The SVF-enriched fat grafting will then be injected in the technique described and diagramed on page 5, section 3. (see figures A and B under section 3). Through 12 incisions placed like the dial of a clock, 1mm Coleman infusion canullas will be used for injecting 1cc of fat in each radial tunnel, putting at least 10–15 tunnels through. The exact same procedure will be done for each breast with equal volumes of SVF-enriched fat.
9. Non-enriched fat grafting cases. In cases were no SVF-enriched fat grafting is done (control group in the Level II study and the contralateral breast in the Level I study), the following protocol will be followed:
A. The syringe liposuction fat harvest will be done in an identical procedure to that done for the SVF-enriched fat grafting procedure.
B. The fat will be processed in identical manner to that done in step 4A on page 12.
C. The fat injection will be done in identical manner to that described in section 6 above (12 incisions placed like the dial of a clock, 1mm tunnels with 1cc injected per tunnel, 10–15 tunnels above the breast parenchyma and 10–15 tunnels below the breast parenchyma.
D. For the control group in the level II study and the contra lateral breast (control breast) in the level I study, less total fat will need to be harvested, since there will be no fat lost (no SVF prepartion) in the level II group and less fat lost (1/2 as much needed for 1 breast SVF preparation) in the level I group.
10. Operative Evaluations

11. Treatment of post op oil filled cyts. Any oil filled cysts found on ultrasound at 3 months will be treated the way that Dr. Yoshimura recommends. If they are smaller than 5mm, they will be left alone. If they are larger than 5mm, they will be aspirated at the same ultrasound imaging procedure by the radiologist, using ultrasound guidance.
12. Data Analysis and Data Monitoring. The collected data for SVF-enriched fat grafting will be compared with contra-lateral breast of the same individual for Level 1 study. For Level II study, the data for SVF-enriched fat grafting will be compared with that of AFG alone control group. All statistical analyses will be performed using Student’s t test and P<0.05 will be defined as significant. The following specific comparisons will be made.
1. Patient satisfaction — This will be documented by a questionnaire and by physician
physical exam using the QOL scale on the questionnaire, and a 1–5 scoring system on
physician physical exam.
2. Breast volume — This will be evaluated based on patients own description of bra cup
size changes, 3D imaging, volumetric measurements, and MRI
3. Softness — This will be evaluated by the patient on a 1–5 scoring system and by the
physician physical exam using the same scale. Here is the scale definition:
I- breast soft as before surgery, no hard areas.
II — breast harder than before surgery, but no palpable fat necrosis or cyts
III- breast harder than before surgery, palpable fat necrosis or cyts — less than 3
palpable lesions
IV — breast harder than before surgery, palpable fat necrosis or cysts — 3–5 palpable
lesions
V — breast harder than before surgery, palpable fat necrosis or cysts → 5 palpable
lesions
4. Subjective symmetry — This will be done with both the Level I and Level II study
groups as follows:
I — breasts symmetric by patient opinion and by physician physical exam
II — breasts mildly asymmetric by patient opinion and/or physician physical exam
III — breasts moderaly asymmetric by patient opinion and/or physician physical
exam
IV — breasts severely asymmetric by patient opinion and/or physician physical
exam
5. Objective volume symmetry — Objective volume symmetry will be evaluated by
volumetric measurements (VM) and by 3D photography for all Level I and Level II
patients. The following system will be used to categorize these differences:
I — less than 5% asymmetry (in ccs) between left and right side by VM and 3D
imaging
II — 5–10% asymmetry (in ccs) between L and R side by VM and 3D imaging
III — 10–20% asymmetry (in ccs) between L and R side by VM and 3D imaging
IV → 20% volume asymmetry (in ccs) between L and R side by VM and 3D
imaging
6. Volume increase — Subjective and Objective volume increase will be evaluated by the
patient, physician physical exam, volumetric imaging, 3D imaging, and MRI. The
following grading system will be used:
Subjective volume increase — patient — grade I — IV system, based on subjective
assessment, using standard photos (pre and post op) and bra cup size change
Physican — grade I- IV system based on subjective size increase, using standard photos and 3D imaging
Objective volume increase — 3D imaging — computer assisted measurement of
3D imaging will be done to estimate volume increase in size of breast
- Volumetric measurements — quantitative changes
in breast volume (in ccs) will be done using
volumetric measurements
- MRI measurements — quantitative changes in breast
volume (in ccs) will be done using computer assisted
3D reformatting of MRI data
1. Mammographic and ultrasound artifacts — ultrasound done at 3 months and
mammography doneat 12 months will be evaluated for surgical artifacts.
The difference in the number of artifacts between autologous fat grafted breasts and
SVF-enriched fat grafted breasts will be compared.
8. Fat-water fraction — Fat to water fraction of augmented breast will be measured
using both proton MR spectroscopy and 3D IDEAL fat-water MRI [145,146]
13. Data Storage and Confidentiality. The research team will insure that strong, state-of-the-art
security is in place to insure the confidentiality of data relating to research participants. All data
and patient information will be saved in both hard copies and computer electronic files. All hard
copies will be locked in a secure cabinet and all computer files will be password protected. All
identifiers will be stripped from the stored samples or computer data, so that they can never be
traced to the individual. If the need to link data to the individual is time limited, stripping
identifiers (rendering the samples truly anonymous) is appropriate once the time window
of need has closed.
I. RISK/BENEFIT ASSESSMENT
1. Risk Category
Minimal risk, which means that the probability and magnitude of harm or discomfort anticipated in the research are not greater than those during the performance of routine surgery of autologous fat breast augmentation.
2. Potential Risk
There is minimal potential risk associated with this clinical trial. Physically, the operation cause no further harm or discomfort than surgery of autologous fat breast augmentation. Psychologically, the cell injection is autologous, which means the cells are obtained from the same individual to which they will be re-implanted. So we believe the procedure will not cause any physiological or psychological disturbance for patients.
3. Protection against Risks
Only appropriate subjects will be enrolled in the trial. Estimated total time for liposuction will be done within 60 minutes to minimize discomfort and stress. Local anesthesia will apply for to avoid pain. During the process of isolating and culture expansion ADSC, all steps will be done in FDA registered facility to minimize the possibility of contamination. Adequate follow-up will be applied to every subject.
4. Potential Benefits to the Subjects
Theoretically subjects will experience larger and better looking breasts after the surgery.
5. Alternatives to Participation
Alternative therapies, such as routine/regular care without SVF injection, are available for those patients who elect not to participate in the study.
II. SUBJECT IDENTIFICATION, RECRUITMENT AND CONSENT
1. Method of Subject Identification and Recruitment.
Recruitment of patients (volunteers) into the study will be done via print media, direct mail, and e-mail announcements. The identification of every subject will be supervised directly by PI and co-PI. The identification and recruitment of subjects will protect privacy and be free of undue influence. No compensation will be given to referring physicians, to patient volunteers, or to any referral service, to avoid any undue influence.
2. Process of Consent
Consent process will be done in a private room for each patient. PI, co-PI, sub-investigator or study coordinator will explain the study to potential subjects in details including risks and benefits, and PI, co-PI, sub-investigator or study coordinator are available to answer any questions the subject may have. There will be sufficient opportunity for the subjects to consider whether or not to participate in the study. Subjects (or subject’s legally authorized representative) will be allowed to take home the unsigned consent forms to think about it for at least 24 hours before signing it.
3. Subject Capacity.
We will only enroll the patients who have the capacity to give informed consent. The patients who do not have the capacity to give informed consent will be excluded from this study.
4. Subject/Representative Comprehension.
We understand that as investigators, we have a legal and ethical obligation to ensure that prospective subjects or subjects’ representatives have sufficient knowledge and comprehension of the information represented by the elements of informed consent to enable them to make an informed and enlightened decision whether or not to participate or allow participation in research. No children or decisional impaired adults will be subjects in this study.
5. Consent Forms.
Consent Form is attached.
6. Documentation of Consent.
The PI and co-PI are responsible for ensuring that valid consent is obtained and documented for all subjects. Consent forms will be documented in locked file cabinet exclusive used for this study only.
7. Costs to the Subject.
The cost of SVF isolation, fat harvest, and AFG will be covered by the patient, except in the Level I study where the patient will received the SVF isolation in one breast without charge.
8. Payment for Participation.
No monetary compensation will be given to study subjects.
9. Data Safety and Monitoring Board (DSMB)
We will develop a DSMB that will be comprised of 4 member-experts who are not engaged in the present study. These four individuals will be academic physicians with expertise in plastic surgery, diseases of the breast, etc. One of the four will be appointed as the chairman of the board. The DSMB will meet with the investigative team twice annually. One of these annual meetings will be in person and the other will be via teleconference. The PI and the Chairman of the DSMB will develop the agenda of each of the DSMB meetings. All researchers and DSMB members that are available will meet during the first and major portion of the meeting. The DSMB members will meet alone in a private session during the second component of the meeting to discuss the generation of their report and the third component of the meeting will consist of the DSMB meeting with the Executive committee of the study (PI, CoPI, and Director of Medical Research at the HPMC).
Bibliography
- Amar O, Bruant-Rodier C, Lehmann S, Bollecker V, Wilk A. Fat tissue transplant: Restoration of the mammary volume after conservative treatment of breast cancers, clinical and radiological considerations. Ann Chir Plast Esthet. 2008 Apr;53(2):169–77. Epub 2007 Oct 23.
- Atik, B., Ozturk, G., Erdogan, E. et al. Comparison of techniques for long-term storage of fat grafts: an experimental study. Plast. Reconstr. Surg 118: 1533, 2006.
- Aygit, A. C., Sarikaya, A., Doganay, L. et al. The fate of intramuscularly injected fat autografts: an experimental study in rabbits. Aesthetic Plast. Surg 28: 334, 2004.
- Baran, C. N., Celebioglu, S., Sensoz, O. et al. The behavior of fat grafts in recipient areas with enhanced vascularity. Plast. Reconstr. Surg 109: 1646, 2002.
- Berman, M. Rejuvenation of the upper eyelid complex with autologous fat transplantation. Dermatol. Surg 26: 1113, 2000.
- Bernard, R. W. and Beran, S. J. Autologous fat graft in nipple reconstruction. Plast. Reconstr. Surg 112: 964, 2003.
- Bertossi, D., Zancanaro, C., Trevisiol, L. et al. Lipofilling of the lips. Arch Facial Plastic Surg 5: 392, 2003.
- Bircoll, M. Cosmetic breast augmentation utilizing autologous fat and liposuction techniques. Plast. Reconstr. Surg 79: 267, 1987.
- Bishop, M. L., Fody, E. P., Schoeff, L. Clinical Chemistry: Principles, Procedures, Correlations. Lippincott, Williams & Wilkins, 2004.
- Brown FE, Sargent SK, Cohen SR, Morain WD. Mammographic changes following reduction mammaplasty. Plast Reconstr Surg. 1987;80(5):691–698.
- Burnouf, M., Buffet, M., Schwarzinger, M. et al. Evaluation of Coleman lipostructure for treatment of facial lipoatrophy in patients with human immunodeficiency virus and parameters associated with the efficiency of this technique. Arch Dermatol. 141: 1220, 2005.
- Butterwick, K. J. Lipoaugmentation for aging hands: a comparison of the longevity and aesthetic results of centrifuged versus noncentrifuged fat. Dermatol. Surg 28: 987, 2002.
- Butterwick, K. J., Bevin, A. A., and Iyer, S. Fat transplantation using fresh versus frozen fat: a side-by-side two-hand comparison pilot study. Dermatol. Surg 32: 640, 2006.
- Canady, J. W., Thompson, S. A., Moon, J. B. et al. Augmentation of oral tissues in rabbit using autogenous fat. Cleft Palate Craniofac. J 32: 1, 1995.
- Cardenas, J. C. and Carvajal, J. Refinement of rhinoplasty with lipoinjection. Aesthetic Plast. Surg 31: 501, 2007.
- Castello, J. R., Barros, J., and Vazquez, R. Giant liponecrotic pseudocyst after breast augmentation by fat injection. Plast. Reconstr. Surg 103: 291, 1999.
- Clavijo-Alvarez, J. A., Rubin, J. P., Bennett, J. et al. A novel perfluoroelastomer seeded with adipose-derived stem cells for soft-tissue repair. Plast. Reconstr. Surg 118: 1132, 2006.
- Coleman, S. R. and Saboeiro, A. P. Fat grafting to the breast revisited: safety and efficacy. Plast. Reconstr. Surg 119: 775, 2007.
- Coleman, S. R. Hand rejuvenation with structural fat grafting. Plast. Reconstr. Surg 110: 1731, 2002.
- Coleman, S. R. Lower Lid Deformity Secondary to Autogenous Fat Transfer: A Cautionary Tale. Aesthetic Plast. Surg 2007.
- Coleman, S. R. Structural fat grafting: more than a permanent filler. Plast. Coleman, S. R. Structural fat grafting: more than a permanent filler. Plast. Reconstr. Surg 118: 108S, 2006.
- Cortese, A., Savastano, G., and Felicetta, L. Free fat transplantation for facial tissue augmentation. J Oral Maxillofac. Surg 58: 164, 2000.
- Dasiou-Plakida, D. Fat injections for facial rejuvenation: 17 years experience in 1720 patients. J Cosmet. Dermatol. 2: 119, 2003.
- Domergue, S., Psomas, C., Yachouh, J. et al. Fat microinfiltration autografting for facial restructuring in HIV patients. J Craniomaxillofac. Surg 34: 484, 2006.
- Duskova, M. and Kristen, M. Augmentation by autologous adipose tissue in cleft lip and nose. Final esthetic touches in clefts: part I. J Craniofac. Surg 15: 478, 2004.
- Ellenbogen, R., Motykie, G., Youn, A. et al. Facial Reshaping Using Less Invasive Methods. Aesthetic Surg J 25: 144, 2005.
- Ellenbogen, R., Youn, A., Yamini, D. et al. The Volumetric Face Lift. Aesthetic Surg J 2004 24: 514, 2004.
- Erol, O. O. Facial autologous soft-tissue contouring by adjunction of tissue cocktail injection (micrograft and minigraft mixture of dermis, fascia, and fat). Plast. Reconstr. Surg 106: 1375, 2000.
- Ersek, R. A., Chang, P., and Salisbury, M. A. Lipo layering of autologous fat: an improved technique with promising results. Plast. Reconstr. Surg 101: 820, 1998.
- Fagrell, D., Eneström, S. et al. Fat cylinder transplantation: an experimental comparative study of three different kinds of fat transplants. Plast. Reconstr. Surg 98: 90, 1996.
- Feinendegen, D. L., Baumgartner, R. W., Schroth, G. et al. Middle cerebral artery occlusion AND ocular fat embolism after autologous fat injection in the face. J Neurol. 245: 53, 1998.
- Fraser J, Wulur I, Alfonso Z, et al. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol 2006;4:150–154.
- Fulton, J. E. Breast contouring with “gelled” autologous fat: A 10-year update. International Journal of Cosmetic Surgery and Aesthetic Dermatology 5: 155, 2003.
- Fulton, J. E., Jr., Rahimi, A. D., Helton, P. et al. Lip rejuvenation. Dermatol. Surg 26: 470, 2000.
- Gimble J, Katz A, Bunnell A. Adipose-derived stem cells for regenerative medicine. Circ Res 2007;100:1249–1260.
- Goehde, S. C., Kuehl, H., and Ladd, M. E. Magnetic resonance imaging of autologous fat grafting. Eur. Radiol. 15: 2423, 2005.
- Gonzalez, A. M., Lobocki, C., Kelly, C. P. et al. An alternative method for harvest and processing fat grafts: an in vitro study of cell viability and survival. Plast. Reconstr. Surg 120: 285, 2007.
- Guaraldi, G., De, F. D., Orlando, G. et al. Facial lipohypertrophy in HIV-infected subjects who underwent autologous fat tissue transplantation. Clin Infect. Dis. 40: e13-e15, 2005.
- Guaraldi, G., Orlando, G., De, F. D. et al. Comparison of three different interventions for the correction of HIV-associated facial lipoatrophy: a prospective study. Antivir. Ther.10: 753, 2005.
- Guerrerosantos, J. Simultaneous rhytidoplasty and lipoinjection: a comprehensive aesthetic surgical strategy. Plast. Reconstr. Surg 102: 191, 1998.
- Guerrerosantos, J., Gonzalez-Mendoza, A., Masmela, Y. et al. Long-term survival of free fat grafts in muscle: an experimental study in rats. Aesthetic Plast. Surg 20: 403, 1996.
- Guyuron, B. and Majzoub, R. K. Facial augmentation with core fat graft: a preliminary report. Plast. Reconstr. Surg 120: 295, 2007.
- Haik, J., Talisman, R., Tamir, J. et al. Breast augmentation with fresh-frozen homologous fat grafts. Aesthetic Plast. Surg 25: 292, 2001.
- Hang-Fu, L., Marmolya, G., and Feiglin, D. H. Liposuction fat-fillant implant for breast augmentation and reconstruction. Aesthetic Plast. Surg 19: 427, 1995.
- Harrison, D. and Selvaggi, G. Gluteal augmentation surgery: indications and surgical management. J Plast. Reconstr. Aesthet. Surg 60: 922, 2007.
- Hu, S., Zhang, H., Feng, Y. et al. Introduction of an easy technique for purification and injection of autogenous free fat parcels in correcting of facial contour deformities. Ann. Plast. Surg 58: 602, 2007.
- Huss, F. R. and Kratz, G. Adipose tissue processed for lipoinjection shows increased cellular survival in vitro when tissue engineering principles are applied. Scand. J Plast. Reconstr. Surg Hand Surg 36: 166, 2002.
- Jackson, I. T., Simman, R., Tholen, R. et al. A successful long-term method of fat grafting: recontouring of a large subcutaneous postradiation thigh defect with autologous fat transplantation. Aesthetic Plast. Surg 25: 165, 2001.
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature July 4, 2002. 418: 41–49.
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. June 14, 2007. 447: 880–881.
- Kanchwala, S. K., Holloway, L., and Bucky, L. P. Reliable soft tissue augmentation: a clinical comparison of injectable soft-tissue fillers for facial-volume augmentation. Ann. Plast. Surg 55: 30, 2005.
- Karabulut, A. B. and Tumerdem, B. Obtaining predictable results in malar augmentation with preimplant fat injection. Plast. Reconstr. Surg 114: 1974, 2004.
- Karacal, N., Cobanoglu, U., Ambarcioglu, O. et al. The effect of fibrin glue on fat graft survival. J Plast. Reconstr. Aesthet. Surg 60: 300, 2007.
- Karacalar, A., Orak, I., Kaplan, S. et al. No-touch technique for autologous fat harvesting. Aesthetic Plast. Surg 28: 158, 2004.
- Karacaoglu, E., Kizilkaya, E., Cermik, H. et al. The role of recipient sites in fat-graft survival: experimental study. Ann. Plast. Surg 55: 63, 2005.
- Kaufman, M. R., Bradley, J. P., Dickinson, B. et al. Autologous fat transfer national consensus survey: trends in techniques for harvest, preparation, and application, and perception of short- and long-term results. Plast. Reconstr. Surg 119: 323, 2007.
- Kim WS, Park BS, Sung JH, The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opinion on Biological Therapy, July 2009, 9(7): 879–887.
- Kneeshaw PJ, Lowry M, Manton D, Hubbard A, Drew PJ, Turnbull LW. Differentiation of benign from malignant breast disease associated with screening detected micro calcifications using dynamic contrast enhanced MRI. Breast. 2006;15(1):29–38.
- Kuran, I. and Tumerdem, B. A new simple method used to prepare fat for injection. Aesthetic Plast. Surg 29: 18, 2005.
- Kwak, J. Y., Lee, S. H., Park, H. L. et al. Sonographic findings in complications of cosmetic breast augmentation with autologous fat obtained by liposuction. J Clin Ultrasound 32: 299, 2004.
- Lacy, E. L. and Bartness, T. J. Autologous fat transplants influence compensatory white adipose tissue mass increases after lipectomy. Am. J Physiol Regul. Integr. Comp Physiol 286: R61-R70, 2004.
- Lacy, E. L. and Bartness, T. J. Effects of white adipose tissue grafts on total body fat and cellularity are dependent on graft type and location. Am. J Physiol Regul. Integr. Comp Physiol 289: R380-R388, 2005.
- Lalikos, J. F., Li, Y. Q., Roth, T. P. et al. Biochemical assessment of cellular damage after adipocyte harvest. J Surg Res. 70: 95, 1997.
- Latoni, J. D., Marshall, D. M., and Wolfe, S. A. Overgrowth of fat autotransplanted for correction of localized steroid-induced atrophy. Plast. Reconstr. Surg 106: 1566, 2000.
- Lee, P. E., Kung, R. C., and Drutz, H. P. Periurethral autologous fat injection as treatment for female stress urinary incontinence: a randomized double-blind controlled trial. J Urol.165: 153, 2001.
- Lidagoster, M. I., Cinelli, P. B., Levee, E. M. et al. Comparison of autologous fat transfer in fresh, refrigerated, and frozen specimens: an animal model. Ann. Plast. Surg 44: 512, 2000.
- MacRae, J. W., Tholpady, S. S., Ogle, R. C. et al. Ex vivo fat graft preservation: effects and implications of cryopreservation. Ann. Plast. Surg 52: 281, 2004.
- Miller, J. J. and Popp, J. C. Fat hypertrophy after autologous fat transfer. Ophthal. Plast. Reconstr. Surg 18: 228, 2002.
- Missana, M. C., Laurent, I., Barreau, L. et al. Autologous fat transfer in reconstructive breast surgery: indications, technique and results. Eur. J Surg Oncol. 33: 685, 2007.
- Monreal, J. Fat tissue as a permanent implant: new instruments and refinements. Aesthetic Surg J 23: 213, 2003.
- Moscatello, D. K., Dougherty, M., Narins, R. S. et al. Cryopreservation of human fat for soft tissue augmentation: viability requires use of cryoprotectant and controlled freezing and storage. Dermatol. Surg 31: 1506, 2005.
- Murillo, W. L. Buttock augmentation: case studies of fat injection monitored by magnetic resonance imaging. Plast. Reconstr. Surg 114: 1606, 2004.
- Nakagami H, Morishita R, Maeda K, Kikuchi Y, Ogihara T, Kaneda Y. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J AtherosclerThromb 2006;13(2):77–81.
- Narins, R. S., Tope, W. D., Pope, K. et al. Overtreatment effects associated with a radiofrequency tissue-tightening device: rare, preventable, and correctable with subcision and autologous fat transfer. Dermatol. Surg 32: 115, 2006.
- Nathan S, Das De S, Thambyah A, Fen C, Goh J, Lee EH. Cell-based therapy in the repair of osteochondral defects: a novel use for adipose tissue. Tissue Eng 2003;9(4):733–744.
- Niechajev, I. Lip enhancement: surgical alternatives and histologic aspects. Plast. Reconstr. Surg 105: 1173, 2000.
- Nishimura, T., Hashimoto, H., Nakanishi, I. et al. Microvascular angiogenesis and apoptosis in the survival of free fat grafts. Laryngoscope 110: 1333, 2000.
- Özsoy, Z., Kul, Z., and Bilir, A. The Role of Cannula Diameter in Improved Adipocyte Viability: A Quantitative Analysis. Aesthetic Surg J 26: 287, 2006.
- Panfilov, D. E. Augmentative phalloplasty. Aesthetic Plast. Surg 30: 183, 2006.
- Parker A, Katz A. Adipose-derived stem cells for the regeneration of damaged tissues. Expert Opin Biol Ther 2006;6:567–578.
- Piasecki, J. H., Gutowski, K. A., Lahvis, G. P. et al. An experimental model for improving fat graft viability and purity. Plast. Reconstr. Surg 119: 1571, 2007.
- Pierrefeu-Lagrange, A. C., Delay, E., Guerin, N. et al. [Radiological evaluation of breasts reconstructed with lipomodeling]. Ann. Chir Plast. Esthet. 51: 18, 2006.
- Pontius, A. T. and Williams, E. F., III The evolution of midface rejuvenation: combining the midface-lift and fat transfer. Arch. Facial. Plast. Surg 8: 300, 2006.
- Pu, L. L. Q., Cui, X., Li, J. et al. The fate of cryopreserved adipose aspirates after in vivo transplantation. Aesthetic Surg J 26: 653, 2006.
- Pu, L. L., Cui, X., Fink, B. F. et al. Adipose aspirates as a source for human processed lipoaspirate cells after optimal cryopreservation. Plast. Reconstr. Surg 117: 1845, 2006.
- Pu, L. L., Cui, X., Fink, B. F. et al. The viability of fatty tissues within adipose aspirates after conventional liposuction: a comprehensive study. Ann. Plast. Surg 54: 288, 2005.
- Pulagam, S. R., Poulton, T., and Mamounas, E. P. Long-term clinical and radiologic results with autologous fat transplantation for breast augmentation: case reports and review of the literature. Breast J 12: 63, 2006.
- Rehman, J., Traktuev, D., Li, J. et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109: 1292, 2004.
- Reiche-Fischel, O., Wolford, L. M., and Pitta, M. Facial contour reconstruction using an autologous free fat graft: a case report with 18-year follow-up. J Oral Maxillofac. Surg 58: 103, 2000.
- Restrepo, J. C. C. and Ahmed, J. A. M. Large-volume lipoinjection for gluteal augmentation. Aesthetic Surg J 22: 33, 2002.
- Ricaurte, J. C., Murali, R., and Mandell, W. Uncomplicated postoperative lipoid meningitis secondary to autologous fat graft necrosis. Clin Infect. Dis. 30: 613, 2000.
- Rieck, B. and Schlaak, S. In vivo tracking of rat preadipocytes after autologous transplantation. Ann. Plast. Surg 51: 294, 2003.
- Rieck, B. and Schlaak, S. Measurement in vivo of the survival rate in autologous adipocyte transplantation. Plast. Reconstr. Surg 111: 2315, 2003.
- Rigotti G, Marchi A, Galiè M, Baroni G, Benati D, Krampera M, Pasini A, Sbarbati A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119(5):1409–1422; discussion 1423–1424.
- Rigotti, G., Marchi, A., Galie, M. et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast. Reconstr. Surg 119: 1409, 2007.
- Roberts, T. L., Toledo, L. S., and Badin, A. Z. Augmentation of the Buttocks by Micro Fat Grafting. Aesthetic Surg J 21: 311, 2001.
- Rohrich, R. J., Sorokin, E. S., and Brown, S. A. In search of improved fat transfer viability: a quantitative analysis of the role of centrifugation and harvest site. Plast. Reconstr. Surg 113: 391, 2004.
- Rose, J. G., Jr., Lucarelli, M. J., Lemke, B. N. et al. Histologic comparison of autologous fat processing methods. Ophthal. Plast. Reconstr. Surg 22: 195, 2006.
- Rubin, J. P., Bennett, J. M., Doctor, J. S. et al. Collagenous microbeads as a scaffold for tissue engineering with adipose-derived stem cells. Plast. Reconstr. Surg 120: 414, 2007.
100. Sadick, N. S. and Hudgins, L. C. Fatty acid analysis of transplanted adipose tissue. Arch. Dermatol. 137: 723, 2001.
- Samdal, F., Skolleborg, K. C., and Berthelsen, B. The effect of preoperative needle abrasion of the recipient site on survival of autologous free fat grafts in rats. Scand. J Plast. Reconstr. Surg Hand Surg 26: 33, 1992.
- Sardesai, M. G. and Moore, C. C. Quantitative and qualitative dermal change with microfat grafting of facial scars. Otolaryngol. Head Neck Surg 137: 868, 2007.
- Schaffler A, Buchler C. Concise review: adipose tissuederived stem cells — basic and clinical implications for novel cell-based therapies. Stem Cells 2007;25:818–827.
- Serra-Renom, J. M. and Fontdevila, J. Treatment of facial fat atrophy related to treatment with protease inhibitors by autologous fat injection in patients with human immunodeficiency virus infection. Plast. Reconstr. Surg 114: 551, 2004.
- Shiffman, M. A. and Mirrafati, S. Fat transfer techniques: the effect of harvest and transfer methods on adipocyte viability and review of the literature. Dermatol. Surg 27: 819, 2001.
- Shoshani, O., Berger, J., Fodor, L. et al. The effect of lidocaine and adrenaline on the viability of injected adipose tissue — an experimental study in nude mice. J Drugs Dermatol. 4: 311, 2005.
- Shoshani, O., Shupak, A., Ullmann, Y. et al. The effect of hyperbaric oxygenation on the viability of human fat injected into nude mice. Plast. Reconstr. Surg 106: 1390, 2000.
- Shoshani, O., Ullmann, Y., Shupak, A. et al. The role of frozen storage in preserving adipose tissue obtained by suction-assisted lipectomy for repeated fat injection procedures. Dermatol. Surg 27: 645, 2001.
- Smith, P., Adams, W. P., Jr., Lipschitz, A. H. et al. Autologous human fat grafting: effect of harvesting and preparation techniques on adipocyte graft survival. Plast. Reconstr. Surg 117: 1836, 2006.
- Spear, S. L., Wilson, H. B., and Lockwood, M. D. Fat injection to correct contour deformities in the reconstructed breast. Plast. Reconstr. Surg 116: 1300, 2005.
- Spyropoulos, E., Christoforidis, C., Borousas, D. et al. Augmentation phalloplasty surgery for penile dysmorphophobia in young adults: considerations regarding patient selection, outcome evaluation and techniques applied. Eur. Urol. 48: 121, 2005.
- Stashower, M., Smith, K., Williams, J. et al. Stromal progenitor cells present within liposuction and reduction abdominoplasty fat for autologous transfer to aged skin. Dermatol. Surg 25: 945, 1999.
- Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, Hedrick MH. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med 2005;54(3):132–410.
- Tholpady SS, Llull R, Ogle RC, et al. Adipose tissue: stemcells and beyond. Clin Plast Surg 2006;33(1):55–62.
- To, W. C., Seeley, B. M., Castor, S. A. et al. One-year survival of AlloDerm allogenic sermal graft and fat autograft in lip augmentation. Aesthetic Surg J 22: 349, 2002.
- Torio-Padron, N., Baerlecken, N., Momeni, A. et al. Engineering of adipose tissue by injection of human preadipocytes in fibrin. Aesthetic Plast. Surg 31: 285, 2007.
- Valdatta, L., Thione, A., Buoro, M. et al. A case of life-threatening sepsis after breast augmentation by fat injection. Aesthetic Plast. Surg 25: 347, 2001.
- Witort, E. J., Pattarino, J., Papucci, L. et al. Autologous lipofilling: coenzyme Q10 can rescue adipocytes from stress-induced apoptotic death. Plast. Reconstr. Surg 119: 1191, 2007.
- Wolter, T. P., von, H. D., Stoffels, I. et al. Cryopreservation of mature human adipocytes: in vitro measurement of viability. Ann. Plast. Surg 55: 408, 2005.
- Yamaguchi, M., Matsumoto, F., Bujo, H. et al. Revascularization determines volume retention and gene expression by fat grafts in mice. Exp. Biol. Med (Maywood. ) 230: 742, 2005.
- Yazawa, M., Mori, T., Tuchiya, K. et al. Influence of vascularized transplant bed on fat grafting. Wound. Repair Regen. 14: 586, 2006.
- Yi, C. G., Xia, W., Zhang, L. X. et al. VEGF gene therapy for the survival of transplanted fat tissue in nude mice. J Plast. Reconstr. Aesthet. Surg 60: 272, 2007.
- Yi, C., Pan, Y., Zhen, Y. et al. Enhancement of viability of fat grafts in nude mice by endothelial progenitor cells. Dermatol. Surg 32: 1437, 2006.
- Yoon, S. S., Chang, D. I., and Chung, K. C. Acute fatal stroke immediately following autologous fat injection into the face. Neurology 61: 1151, 2003.
- Yoshimura, K., Sato, K., Aoi, N. et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast. Surg 32: 48, 2008.
- Yunud M, Ahmed N, Masroor I, Yagoob J. Mammographic criteria for determining the diagnostic value of micro-calcifications in the detection of early breast cancer. J Pak Med Assoc. 2004;54(1):24–29.
- Zuk PA. The Adipose-derived Stem Cell: Looking Back and Looking Ahead. Mol Biol Cell. 2010 Jun;21(11):1783–7. Epub 2010 Apr 7.
Yoshimura group papers:
- Yoshimura K, Asano Y, Aoi N, Kurita M, Oshima Y, Sato K, Inoue K, Suga H, Eto H, Kato H, Harii K. Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J. 2010 Mar-Apr;16(2):169–75. Epub 2009 Nov 12.
- Suga H, Eto H, Aoi N, Kato H, Araki J, Doi K, Higashino T, Yoshimura K. Adipose tissue remodeling under ischemia: death of adipocytes and activation of stem/progenitor cells. Plast Reconstr Surg. 2010 Dec;126(6):1911–23.
- Kato H, Suga H, Eto H, Araki J, Aoi N, Doi K, Iida T, Tabata Y, Yoshimura K. Reversible adipose tissue enlargement induced by external tissue suspension: possible contribution of basic fibroblast growth factor in the preservation of enlarged tissue. Tissue Eng Part A. 2010 Jun;16(6):2029–40.
- Eto H, Suga H, Matsumoto D, Inoue K, Aoi N, Kato H, Araki J, Yoshimura K. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast Reconstr Surg. 2009 Oct;124(4):1087–97.
- Yoshimura K, Suga H, Eto H. Adipose-derived stem/progenitor cells: roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regen Med. 2009 Mar;4(2):265–73. Review.
- Suga H, Araki J, Aoi N, Kato H, Higashino T, Yoshimura K. Adipose tissue remodeling in lipedema: adipocyte death and concurrent regeneration. J Cutan Pathol. 2009 Dec;36(12):1293–8.
- Suga H, Matsumoto D, Eto H, Inoue K, Aoi N, Kato H, Araki J, Yoshimura K. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 2009 Oct;18(8):1201–10.
- Yoshimura K, Sato K, Aoi N, Kurita M, Inoue K, Suga H, Eto H, Kato H, Hirohi T, Harii K. Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008 Sep;34(9):1178–85. Epub 2008 May 29.
- Kurita M, Matsumoto D, Shigeura T, Sato K, Gonda K, Harii K, Yoshimura K. Influences of centrifugation on cells and tissues in liposuction aspirates: optimized centrifugation for lipotransfer and cell isolation. Plast Reconstr Surg. 2008 Mar;121(3):1033–41; discussion 1042–3.
- Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008 Jan;32(1):48–55; discussion 56–7. Epub 2007 Sep 1.
- Matsumoto D, Sato K, Gonda K, Takaki Y, Shigeura T, Sato T, Aiba-Kojima E, Iizuka F, Inoue K, Suga H, Yoshimura K. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006 Dec;12(12):3375–82.
- Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, Gonda K. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006 Jul;208(1):64–76.
Cytori papers:
- Hicok KC, Hedrick MH. Automated isolation and processing of adipose-derived stem and regenerative cells. Methods Mol Biol. 2011;702:87–105.
- Zhu M, Zhou Z, Chen Y, Schreiber R, Ransom JT, Fraser JK, Hedrick MH, Pinkernell K, Kuo HC. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg. 2010 Feb;64(2):222–8.
- Lin K, Matsubara Y, Masuda Y, Togashi K, Ohno T, Tamura T, Toyoshima Y, Sugimachi K, Toyoda M, Marc H, Douglas A. Characterization of adipose tissue-derived cells isolated with the Celution system. Cytotherapy. 2008;10(4):417–26.
- Moseley TA, Zhu M, Hedrick MH. Adipose-derived stem and progenitor cells as fillers in plastic and reconstructive surgery. Plast Reconstr Surg. 2006 Sep;118(3 Suppl):121S-128S. Review.
Publications from Dr. Peter Rubin of the U. of Pittsburgh group:
- Brown BN, Fruend JM, Li H, Rubin PJ, Reing JE, Jeffries EM, Wolf MT, Tottey S, Barnes CA, Ratner B, Badylak SF. Comparison of Three Methods for the Derivation of a Biologic Scaffold Composed of Adipose Tissue Extracellular Matrix. Tissue Eng Part C Methods. 2010 Nov 3.
Other references
- Reeder SB, Pineda AR, Wen Z et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med 2005;54:636–644.
- Hu HH, Kim HW, Nayak KS: Comparison of fat-water MRI and single-voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obesity 2010;18–4: 841–7
Comments
Post a Comment